Nutrition: Conclusions
[addw2p name=”oilPalmNutrition”]
The annual B requirement of oil palm increases rapidly and reaches a peak at six years after planting. B is mainly needed for the canopy development and fresh fruit bunch production. Majority of the absorbed B is phloem immobile but there is an indication that B in the petiole and rachis of old leaf is phloem mobile. By the time of replanting, B immobilized in the stem is sufficient to meet B requirement of oil palm in its first four years of growth and production. A management strategy is therefore required to recycle this substantial amount of B to the next generation of oil palm.
Nutrition: Discussion
[addw2p name=”oilPalmNutrition”]
The B requirement of oil palm in the first 82 months after planting was mainly driven by its growth rate, a process termed as growth demand for nutrients by Tinker and Nye (2000). This is due to the relatively constant B concentration across palm age in each palm component, which is in agreement with the results of Ng et al. (1968). However, the current Tenera oil palm on infertile Oxisols requires about 27 % more B than the older Dura planting material on fertile Inceptisols. This difference might be attributed to the larger canopy of the Tenera oil palm which is responsive to high fertilizer regime (Goh et al ., 2003).
The B content of the roots had not been studied in the oil palm. Initially it partitions a large proportion of its biomass to the roots to maximize the exploitation of soil water and nutrients. This results in relatively large B requirement for root development. But as the other vegetative matter develop and production commences, the proportion of B for roots declines to less than 6 % (Figure 1).
The annual B requirement decreased from the sixth year after planting due mainly to the declining growth rate of the stem (Figure 1). Extrapolating the model for the annual B requirement shows that the steady-state B uptake of 198 g ha-1 yr-1 as estimated using the 16 years old palms in the second experiment would be reached at the twelfth year after planting. This corresponds with the findings of Gerritsma and Soebagyo (1999). They found that the vegetative growth of oil palm reached its maximum at the twelfth year after planting, and FFB yields were relatively stable thereafter. Therefore, the nutrient requirements including B can be anticipated to be in a steady state too.
The Tenera fresh fruit bunches contained 2.39 mg B kg-1 which was only 12 % greater than Dura bunches reported by Ng et al. (1968). This was despite its bigger mesocarp and thinner shell and the former containing more B. This lack of difference could be partially attributed to the higher B concentrations in the stalk and empty spikelets of Dura bunches although strong conclusion could not be made since Ng et al . (1968) analyzed only two bunches compared with 26 bunches in this study.
The B distribution in the vegetative components and FFB of oil palm suggests that B is mainly transported by the xylem. In the oil palm, B is considered to be phloem immobile based on the work of Rajaratnam (1972). Moreover, B toxicity symptoms of oil palm are exhibited first in the tips and margins of leaflets of older leaves suggesting that it is a non-polyol producing plant (Brown et al ., 1999) and therefore, its B has restricted phloem mobility. However, a detailed analysis of the B concentrations in the canopy implies that B may be translocated in the phloem also. The young developing (spear) leaves had B concentration similar to the matured leaves (leaves 9 to 16) and higher than the maturing leaves (leaves 1 to 8). But the older leaves (leaf 33 and older) tended to have the lowest B concentration (Table 5). The declines in B concentration occurred in the petiole and rachis but not the leaflets (Table 6). This indicates that B may be mobile in the phloem of petiole and rachis only. Both Ng et al . (1968) and Rajaratnam (1972) did not investigate the B concentrations in the spear leaves and leaves older than 33. Thus, they did not observe the above and the latter concluded that B was phloem immobile while the former contended that there was no consistent trend. Rajaratnam (1972) further illustrated that B could be lost from the leaves through guttation in order to explain the differences in B contents in the leaves at different time of sampling and leaf age. However, this postulation could not explain our results since guttation can occur in all the leaves.
The oil palm is mainly planted on highly weathered Ultisols and Oxisols with generally low soil B content. Thus, increasing rates of soluble B fertilizer in the first six years after planting are usually required to match the annual B requirements (Figure 1). Apart from the first year, the B rates should range between 2.25 and 4.5 kg B ha-1 yr-1 due to the low fertilizer use efficiency of less than 15 % as obtained in this study. Subsequently, the B rates may be decreased to between 2 and 3 kg B ha-1 yr-1 for palms between seven and twelve years old. At the steady state of 12 years or older, only occasional B applications are probably necessary since B exported out of the system through FFB and immobilized in the stem is relatively low.
About 43 % of the B in FFB is found in the stalk and empty spikelets which can be returned to the fields via empty fruit bunches. It is probably also worthwhile to build-up B in the oil palm canopy in the early years to increase the B content in the leaf pile, which is the dominant source of organic matter in the plantations. This is because organic matter can be an effective source of B by mineralization when the soil B is low (Bell et al ., 2002).
The oil palm accumulates substantial B in its vegetative components and by the time of replanting at 25 years old, it should reach about 750 g B ha-1. This is sufficient to meet the B requirements of oil palm in the first four years. The current zero-burn replanting technique where the palms are felled, chipped and pulzerized, and the organic residues spread throughout the field may be able to return the palm B to the soils although the next generation of oil palms may not be able to exploit it fully due to its initial small root system and the large leaching loss of B in Malaysian soils (Rajaratnam, 1973b).
Nutrition: Results
[addw2p name=”oilPalmNutrition”]
B requirements of the oil palm
The B contents of the canopy and stem increased rapidly from 20 to 82 month after planting (Table 1). These increases were mainly due to palm growth since their B concentrations were relatively constant over the period of measurements. Root B content was highest (44 g ha-1) at 37 months after planting but it quickly stabilized thereafter at an average of 34 g ha-1 (Table 1). The B requirements for FFB yields and production of male inflorescences between the sampling intervals increased exponentially before reaching a plateau at 71 months after planting (Table 1), again due to biomass increments rather than B concentrations in the palm components.
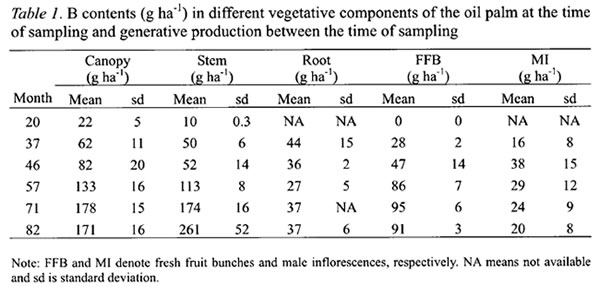
The cumulative B contents for each palm component followed a non-linear regression model (Table 2). The cumulative B contents for canopy and stem fitted well to modified exponential models whereas those for roots and FFB yields to hyperbolic models. The latter implied that the B requirements were relatively constant after their maximum has been attained. The r-squares for all the equations exceeded 0.78.
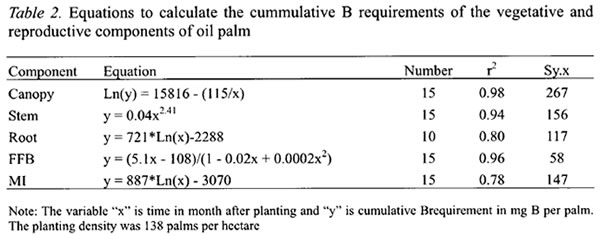
The annual B requirements of oil palm followed a sigmoidal curve with rapid increase from the second year after planting, reaching a maximum of 343 g B ha-1 yr-1 in the sixth year (Figure 1). The annual B requirement then declined to about 220 g B ha-1 yr-1 in the eighth year before stabilizing at 198 g B ha-1 yr-1 from the twelfth year. The latter was based on the estimated annual B requirement of the 16 years old palms at steady state condition in the second experiment. The highest B requirement of oil palm came from its developing canopy, which peaked at the fifth year after planting when the canopies of neighboring palms were fully overlapped (“closed”) and competition began to set in. At the peak, the B requirement for the canopy accounted for more than 40 % of the total B requirement of oil palm.
The B requirement for stem development was also the highest in the sixth year at 67 g B ha-1 yr-1 before declining sharply as palm competition intensified and growth rate slowed down (Figure 1). In contrast, the B requirement for roots was not only the lowest but also attained its peak earlier at the third year after planting (Figure 1). Thereafter, it required about 9 g B ha-1 yr-1 to sustain root turnover or self pruning of roots.
The B requirement for FFB yield was the second largest among the palm components and account for about 33 % of the annual B requirement at the sixth year and increasing to 37 % as the B needs for stem decreased sharply (Figure 1).
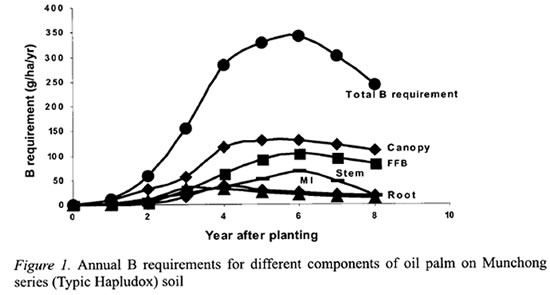
B distribution within the oil palm
The detailed B distribution in the oil palm was studied in the second experiment where the palms were 16 years old and in steady state condition (Tables 3 and 4). The cabbage, which composes mainly meristemic cells, had the highest concentration of B at 12.3 mg kg-1 (Table 3). This was followed by the leaflets which were at the tail-end of the transpiration stream. Interestingly, the petiole, which supports the rachis and leaflets, and the petiole base, which are attached to the stem after the leaf is pruned off, had higher B concentrations than the rachis or stem. The stem and rachis had similar B concentrations of 3.3 and 3.6 mg kg-1, respectively. Although the stem B concentration was low, it has accumulated a large quantity of B by the 16th year at 404 g ha-1. The roots had the lowest B concentration of 1.4 mg kg-1.
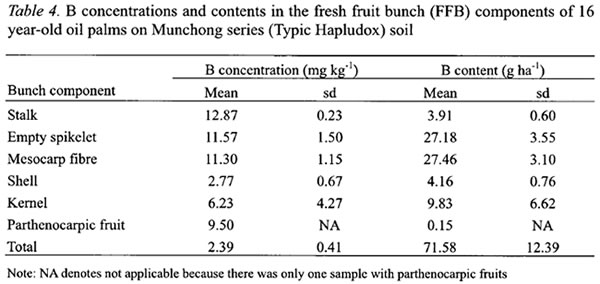
In FFB, larger B concentrations were found in the stalk, empty spikelets and mesocarp fibre (Table 4). These components have more rapid transpiration rate particularly during fruits development and oil formation stages (Jeje et al ., 1978). B concentration in the kernel at 6.23 mg kg-1was 2.25 times more than the shell (Table 4). The stalk and empty spikelets, which are the main components of empty fruit bunches after the milling process, contained about 43 % of the B in FFB.
A detailed analysis of the B distribution in the canopy showed that the B concentrations seemed to be relatively constant from the spear leaves to leaf number 32 before decreasing in the older leaves (Table 5). In terms of B content, there was an initial increase from leaf 1 to leaf 9 due to increasing frond dry weight. The B contents between leaf 9 and leaf 32 were similar at an average 26.3 g B per frond. The B contents then declined linearly between leaf 33 and leaf 48. This decrease was mainly due to lower dry weights and B concentrations of both petiole and rachis (Table 6). There was no clear trend in B concentrations in the leaflets.

B distribution in an oil palm plantation
The B distribution in the various components of one hectare of mature oil palms at stead state indicated that the largest store of B was in the stem at 404 g ha-1 followed by the canopy at 155 g ha-1 (Figure 2). The ground vegetation of mainly grasses and ferns contained very low B at only 6 g ha-1.
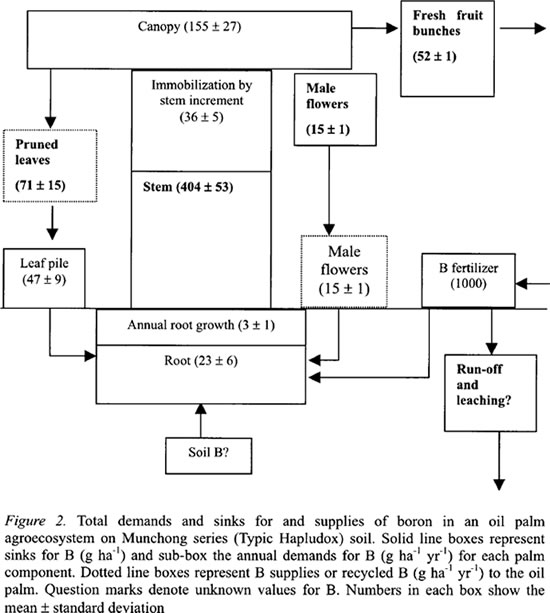
In terms of B cycling, the pruned leaves would return 71 g B ha-1 yr-1 to the agroecosystem and the decaying male inflorescences about 15 g B ha-1 yr-1. The pruned leaves were neatly stacked in leaf piles in the area between the palm rows called the inter-rows. The latter area stored about 47 kg B ha-1, which was 66 % of the annual B in the pruned leaves. The lower B value in the leaf pile might be attributed to the high decomposition rate of oil palm leaf where most of it would decay within six months.
In the oil palm agroecosystem, the FFB yields are transported to the oil palm mill for processing, and this component contained 52 g B ha-1 yr-1. The oil palm also immobilized about 36 g B ha-1 yr-1 mainly for stem growth.
Nutrition: Materials and Methods
[addw2p name=”oilPalmNutrition”]
Experimental sites
The oil palms selected for study came from the optimal NPK treatments of two factorial, fertilizer response trials. Both experiments were conducted on Munchong series (Typic Hapludox) soil which was derived from shale. In the first experiment, destructive samplings of the palms were carried out at six palm ages to study the B requirements of growing palms. Two palms were sampled at 20, 37 and 71 months after field planting whereas three palms were sampled at 46, 57 and 82 months after field planting. At each sampling, the palms were chosen from different replicates and where possible, palms from the same replicates were taken across the months. Sodium borate was applied at an average rate of 3.1 kg B ha-1 yr-1.
In the second experiment, two 16 years old palms, the interrow vegetation and the frond stacks were sampled to examine the B distribution in an oil palm field at the steady state condition. Sodium borate was applied at an average rate of about 1 kg B ha-1 yr-1.
In both experiments, the number of leaves produced by each sampled palm were measured at half yearly intervals, the fresh fruit bunch (FFB) yields at 10 day intervals and the male inflorescences at quarterly intervals. These data were then summarized on an annual basis to estimate the yearly B requirements of oil palm.
Palm destructive sampling and B analysis
The oil palm can be divided into unambiguous morphological components (Tinker and Smilde, 1963). These are: leaf, which can be further separated into leaflets, leaf rachis and leaf petiole, unopened spear leaves, growing point or “cabbage”, stem, roots, male inflorescences and fresh fruit bunches. In this paper, the stem included the petiole bases, which were attached to it after pruning the leaves, and the root bole, which was the growing point for the roots.
Each fresh leaf was cut down (“fresh” being taken to mean that over half the total leaflet area was still green) and counted. Each leaf was then separated into the leaflets, petiole and rachis. The leaflets for nutrient analysis were sampled systematically by taking 1 in 10. The balance of the leaflets was then sampled for determination of fresh and dry weights. The rachis was cut into three equal sections. Each section was divided into three equal parts and a 10 cm section was cut from the middle of each part. One longitudinal half from each section was bulked for fresh and dry weight determination. Leaf petioles from leaves 1 to 6 were divided into two equal parts whereas the older ones into three equal parts. Leaf 1 was the youngest fully opened leaf. A 10 cm section was cut from the middle of each part. One longitudinal half was then taken from each section and bulked for fresh and dry weight determination. For B analysis, the leaflets, rachis and petiole were each bulked for various groups of leaves in the ratio of their dry weights. In the oil palm, each group or whorl of leaves consists of eight leaves i.e. leaves 1 to 8, 9 to 16, 17 to 24, 25 to 32, 33 to 40, 41 to 48 and 49 to 56. The unopened spear leaves were sub-sampled in the same manner as the leaf but their rachis and petiole were not separated because most of them had very short petioles.
The primary roots around the stem were cut to facilitate its felling at ground level. The cabbage was then cut off from the apex of the stem. Two 1/16th sections were sampled. The rest of the stem was divided into six equal sections. The middle 15 cm of each stem section was sampled and two 1/16th sections sub-sampled.
The roots were sampled using the trenching method. Since the palms were planted in an equilateral triangular pattern of 9.1 m and 8.9 m apart for experiment 1 and 2 respectively, the palm area was divided into three sections as follows: palm to palm of the same row, palm to palm of another row and palm to interrow area. In each section, a trench 30 cm wide and 90 cm depth was dug to the middle of the planting row or interrow area. The roots that were dug up were sieved out and collected for every 30 cm by 100 cm by 30 cm depth section and then bulked for each palm
The male infloresecences on the palms at the time of sampling were separated into immature inflorescences, mature inflorescences and old inflorescences. The FFB for B analysis were collected from the second experiment only. A total of 26 bunches were collected over a three month period. In the laboratory, each bunch was weighed, stripped and divided into its major unique components of fruit, stalk, spikelets and parthenocarpic fruits. The fruit was further separated into the shell and kernel. Fresh weight of each component was taken. Two 100 g samples of each of the chopped and ground stalk and spikelets were collected for fresh and dry weight determination. The oil in the fruits were extracted using hexane following the modified Blaak’s method (Rao et al ., 1983).
The aerial parts of the interrow vegetation in each palm area were cut and weighed. The vegetation on the palm stem was also sampled, weighed and bulked with the interrow vegetation. Only one eighth of the fresh weight was taken for analysis.
All sub-samples of the vegetative and reproductive parts were sent to the laboratory for fresh and dry weight determination and B analysis. The latter followed the Azomethine-H method (John et al ., 1975). Briefly, 1 g of plant sample was dry ashed at 530 ° C and digested with 10 ml of 1.4 M H2 SO4. The solution was then filtered through Whatman No. 1 paper. 0.5 ml of 0.05 M EDTA, 1 ml of 0.5 M ammonium acetate and 1 ml of Azomethine-H solution were than added to 1 ml of the filtrate to prevent interferences and developed the colour. The B concentration in the filtrate was then read using a UV/VIS spectrophotometer.
Computing the annual B requirements from the data
In the first experiment, the time intervals were not fixed. Therefore, the cumulative B accumulation or uptake by the palm in each component at each sampling time has to be computed and the results modeled using non-linear regression. The gradient of the model at each yearly time period gave the annual B requirement for the component.
The B requirement of canopy between two sampling periods (t1 and t2) was: B content of new leaves produced between t1 and t2 plus B content accumulated by the older leaves between t1 and t2. It was also assumed that the B content in the canopy at the first sampling represented a continuous accumulation of B from the time of field planting since pruning of the leaves had not commenced then.
The B requirement of roots between two sampling periods (t1 and t2) was computed as follows:
B content at t2 – B content at t1 + a B content at t2
where a is the percentage of self-pruned roots. The a value for each time period was obtained from Jourdan and Rey (1997). It was also assumed that the root biomass continued to grow in the first 36 months after field planting with minimal self pruning following the logistic root model of Jourdan and Rey (1997).
The annual B requirements for fresh fruit bunches and male inflorescences were estimated based on their annual dry matter production multiplied by their average B concentration for the year.
Statistical analysis
Descriptive statistics, means and standard deviations, were calculated using Statistica Ver. 7.0 (StatSoft, 2004). Separate one factor analysis of variance (Anova) was computed for the B distribution within the vegetative components of the palm and the fresh fruit bunches. Non-linear regression was used to model the cummulative vegetative data using CurveExpert 1.38 (Hyams, 2001).
Oil Palm: Nutrition
Boron requirement and distribution in the oil palm (Elaeis guineensis Jacq.) and some implications on manuring practices
[addw2p name=”oilPalmNutrition”]
Oil palm (Elaeis guineensis Jacq.) is grown on more than 12 million hectares in the humid tropics mainly between latitude 10 ° N and 10 ° S. It contributes to about 27 % of the world’s vegetable oils and fats. Oil palm is the world’s most productive oil crop and requires a relatively high amount of B to sustain its growth and production despite being a monocotyledon (Shorrocks, 1997). In fact, it is one of the 16 plants regarded as most sensitive to B deficiency and highly responsive to B application (Shorrocks, 1997).
In Southeast Asia, the oil palm is mainly cultivated on the highly weathered Ultisols and Oxisols derived from granite, sandstones and shales. These soils have low soil B contents (Shorrocks, 1997) and therefore, B deficiency symptoms on the oil palm in various types of malformed, younger leaves are common particularly during drought. Boron deficiency causes premature lignification of the cell walls in the oil palm (Rajaratnam and Lowry, 1974) and under severe conditions, for example, at the little leaf stage, yield may decline by about 83% (Rajaratnam, 1973a). Thus, water-soluble B fertilizer such as Fertibor (15% B) is regularly applied at the rate of 1 to 3 kg B ha-1 yr-1 in the first six years after planting to prevent B deficiency in the oil palm.
Ng et al . (1968), working with the obsolete Dura planting materials, reported that B concentration in the oil palm canopy was similar to the stem. They did not find any trend in B concentrations between leaves of different ages and concluded that B may be mobile in the oil palm. In contrast, Rajaratnam (1972) using the current Tenera planting materials showed increasing B concentrations from the youngest to the oldest leaf suggesting that B is immobile in oil palm. However, they both agreed that B moves rapidly along the transpiration stream resulting in the accumulation of B at the tips of the oil palm leaf and leaflets.
K has been demonstrated to inhibit B uptake by oil palm (Rajaratnam, 1973b) whereas N fertilization may increase palm growth leading to low or deficient B concentration through dilution effect (Shorrocks, 1997). Since then, N and K fertilizer rates for the oil palm have increased substantially (Goh and Kee, 2000). It is therefore important to determine the B requirement of oil palm under current high fertilizer regimes. Tinker and Smilde (1963) postulated that the nutrient requirement of oil palm might be established by analysing the elements in the palm tissues because the bulk of the crop, and consequently its nutrient content, is large, and the long-term nutrient-supplying power of the soil is poor. The fraction of the total available nutrients held in the crop itself can then be considerable, and its nutrient requirements will be related to the amounts immobilized by the plants (Tinker and Smilde, 1963). These conditions are approached by the oil palm growing on soils with poor B supplying capacity as found in most of Southeast Asia. Despite this simple concept, the B requirements and distribution in the oil palm plantation has received little attention in the last three decades and has not been investigated for the current Tenera planting materials except in their canopy. Hence, this study was conducted with the following objectives:
-
To determine the B requirement of oil palm on an Oxisol at different palm ages
-
To investigate B mobility in oil palm using indirect method
-
To examine the distribution of B in an oil palm field at steady state on an Oxisol
Reference
Goh, K.J., Gan, H.H., Kee, K.K., Chew, P.S., and Teoh, K.C., (2007) Boron requirement and distribution in the oil palm (Elaeis guineensis Jacq.) and some implications on manuring practices. In: Xu. F., Goldbach H.E., Brown P.H., Bell R.W., Fujiwara T., Hunt C.D., Goldberg S. (Eds) Advances in Plant and Animal Boron Nutrition, Springer, The Netherlands: 189 – 202.
Note: The full list of references quoted in this article is available from the above paper.
Legume: Mucuna Bracteata
Mucuna bracteata -a cover crop and living green manure (Dr. Chee Kheng Hoy FISP)
Note: This article is written by Dr. Chee in mandarin and published by Agroworld, Issue No. 188, February 2007, Kuala Lumpur: 30-34. Agroworld is a Chinese magazine for farmers. Please read the original article. To subscribe Agroworld, please email or contact Agroworld Enterprise, No. 10, Jalan 3/18D, Taman Mastiara, Off Jalan Ipoh Bt 5 1/2, 51200 Kuala Lumpur, Malaysia. Tel: 603-62500975 / 0972 / 0915
(Translated by Soon, S.H.)
A new leguminous cover crop – Mucuna bracteata is planted in the interrows of rubber and oil palm. The desirable characteristic of this cover that attracts much attention is its ability to produce three to four times more biomass than conventional leguminous cover. Furthermore, it helps to prevent the invasion of pest and diseases in the fields. Many estate managers are unwilling to spend extra money on planting leguminous cover crop. In fact, after the conventional leguminous cover crop grows under open condition for two and a half years, the remaining litter mulch can effectively provide nitrogen for oil palm replanting.
A Mucuna bracteata Seminar was held at Sg. Tekam Plantations Resort, Pahang at the end of 2006 (29 November). During this 2-day seminar, ten papers on Mucuna bracteata ( M. bracteata ) were discussed on the first day while a well-known soil scientist from Malaysia, Dr. S. Paramananthan led us on the identification of different soil profiles on the second day. Param is regarded as an “old friend” to planters from all over the world.

A group photo of the seminar participants who are interested in soil survey. The man in red shirt is Dr. Param, in blue shirt beside him is K.J. Goh, in yellow shirt is C.T. Lee.
Advantages of leguminous cover crop
The seminar ended successfully. Besides the efforts of committee members, we appreciated FELDA support on funds and manpower. The facilities and research programmes of FELDA Sungai Tekam research station as well as the large-scale FELDA oil palm plantations definitely added much charm to the seminar.
The inspiration to organize the seminar comes from my ex-colleague, Mr. Chiu Sheng Bin. Many from the plantation industry know the agronomist, S.B. Chiu. He graduated from Harvard University. He worked as an electrical engineer before he furthered his studies in agriculture. Mr. Chiu and I were invited by agronomist, Lee Chin Tui from FELDA to visit M. bracteata , a leguminous cover crop planted by FELDA on a large scale. Young palms grow in between very well.
Mr. Chiu felt that we should promote the advantages of leguminous cover crop. Unfortunately, he spends most of the time working overseas. When I mentioned about the organization of a cover crop seminar again, besides Lee, Param and Goh Kah Joo also gave their full support to us. Goh was elected as chairman of the seminar. He is Deputy Director of Research of AAR, an associate company of Boustead Plantations Berhad and Kuala Lumpur Kepong Berhad and graduated from University of York with biological computation specialisation. Five of us as committee members (Goh, Param, Lee, Chiu, Chee) had dinner and meeting at the same time at a chinese restaurant in Petaling Jaya twice. We also exchanged opinions through emails.
A senior estate manager, C. Matthews, from Golden Hope imported 2 kg of M. bracteata seeds from India in 1991. This new legume was planted between sapling lines in the rubber plantation in India at that time. Before M. bracteata reached Malaysia, I was already involved in research and development. At that time, I traveled not so far from my work place to a M. bracteata experimental site-Golden Hope Plantation Berhad, North Labis Estate, Johor (please refer to Agroworld magazine Issue No. 114, “New legume produces living green manure”).
Beginning with the new M. bracteata legume at North Labis Estate, Golden Hope Plantations Berhad has now planted 30,000 ha. of this cover crop. Golden Hope is the first plantation company to plant M. bracteata in Sabah. The first company to plant M. bracteata in Indonesia is Lyman Agro. In the early 90’s, Mr. Chiu Sheng Bin and I were doing research and development on oil palm, rubber and forestry in this chinese company. We introduced M. bracteata to Indonesia.

From left : C. Matthews (who is the first to import M. bracteata from India), Z.H. Shamsudin (UPM professor), C.F. Chee (seed supplier), K.J. Goh (seminar chairman), Dr. Chee Kheng Hoy, C.T. Lee (FELDA agronomist), S.B. Chiu (oil palm consultant).
Prevent young palm from invasion of weeds and pests
Many estate managers are unwilling to spend extra money on planting leguminous cover crop. Somehow the cover crop cannot survive under shaded condition after growing for one to two years. In fact, after the conventional leguminous cover crop eg. Pueraria javanica grows under open conditions for two and a half years, the remaining litter mulch can effectively provide nitrogen for the oil palm replant over the following two and a half years.
M. bracteata cover crop can be found in newly planted or replanted oil palm plantations in Malaysia, Indonesia and Colombia, South America today. The desirable characteristic of this cover that attracts much attention is its ability to produce three to four times more biomass (green manure) than conventional leguminous cover. Apart from that, it grows luxuriantly and also has the habit to smother weeds: lallang, shrubs and ferns included.
Rhinoceros beetle damages young palm. The conventional leguminous cover crop can prevent 65% damage while M. bracteata can prevent 93% damage. The thick M. bracteata can physically prevent the invasion of beetle towards the remaining organic residues after oil palm replanting. On the other hand, it provides a moist environment for oil palm residues to decompose faster.
In addition, M. bracteata also interrupts the activities of rat and therefore reduces the rat damage to oil palm.

The seminar participants testify that M. bracteata enhances the oil palm growth at FELDA plantation.
The mulch is beneficial to barren soil
M. bracteata is most impressive as it forms a thick pure cover under oil palm, often 1 m thick with 40 cm of litter mulch below. Even under the shade of 10 years old palms, it still maintains 50 mm of litter mulch below the thick cover of M. bracteata . It produces large amounts of organic matter through its litter mulch and thus rebuilds poor, degraded soils by enriching them with mulch.
M. bracteata seeds do not come cheap. It is about RM 300 per kg, all imported from India. M. bracteata originates from Tripura, Northeast India in the Himalaya range. This area is also known as the northeastern hill region of India and it lies between 21.5-29.5° N latitude and 85.5-97.5° E longitude. Temperature varies between 10 and 35° Celsius and average annual rainfall between 2855 mm and 1811 mm. The day length can be as high as 13.6 hours. Fruits are covered by stinging hairs and turn blackish when ripe during winter only.
From botanical record, Bangladesh, China, Hainan, Laos, Myanmar, Thailand, Vietnam and Andaman Island have M. bracteata growing naturally but there is no record of whether these places produce mucuna seeds.
We had tried to plant M. bracteata in areas with different latitudes and temperature, for instance “Penang Hill” in Penang and highland in Laos. It has flowered but has not seeded yet. The flower emits a stinking smell of rotting meat to attract insects. It is said that one type of insect pollinators that visits the flowers is the hornet.

M. bracteata flowered under a special environment locally. This is the research result of S.B. Chiu. Although it has flowered, but research is still needed on how to make it produces seed.
We need workers to harvest the seed pods produced by M. bracteata planted in our experiment. This task is not easy because seed pods are covered with sharp, needle-like hairs that cause great irritation when touched as they penetrate the skin easily. Perhaps, because of this reason, the workers in India only harvest the seed pods before noon. The weather becomes hot in the afternoon and they return home to “heal their wound”.
To judge the seed quality by condition
One of the papers in the seminar is about the seed quality. The seed quality can be judged by its condition: a well formed seed is big and round, about 4700 seeds per kg, germination rate up to 80%; a slightly undesirable seed is big and flat, has about 7400 seeds per kg, germination rate only 5%; small and round seed contains 11600 seeds per kg, germination rate is about 32%; undesirable seed is small and flat, has about 12000 seeds per kg, germination rate nearly 0%.
A mixture of seeds from the four seed categories above is about 6700 seeds per kg, germination rate is approximately 68%. Briefly, good quality seed should not exceed 7000 seeds per kg, germination rate ranges from 60 to 70%.
The seeds of M. bracteata are similar to another cover crop which will die within a year- Mucuna pruriens . So, planters may easily mistake M. pruriens seeds for those of M. bracteata when purchasing M. bracteata seeds.
There is another type of Mucuna called M. cochinchinensis which is among the various types of leguminous cover crop that are already planted in the estate for a long time. The size of M. cochinchinensis seed is two to three times bigger than M. bracteata . The leaves are somewhat similar but bigger than M. bracteata . M. cochinchinensis is an annual lasting six to seven months. On the other hand, M. bracteata is a perennial although it only grows vigorously after planting for nine to ten months.

M. bracteata grows luxuriantly and vigorously under oil palm shade.
A special edition of the papers that was full of pictures, charts and articles was distributed at the seminar. Some uncommon data and opinions were mentioned. This will be published as a book shortly.
In estates planted with M. bracteata , the soil fertility within 30 cm from the topsoil will be maintained. On the other hand, the soil fertility will decrease continuously in areas full of weeds. This is due to the deep-rooted nature of M. bracteata which might possibly extract nutrients from the deeper layers of the soil and transport it to the vines and leaves and deposit them on the surface in the form of mulch or organic matter after that.
M. bracteata was firstly planted as a cover crop in rubber plantation in India. Larger numbers of bacteria and fungi were counted under M. bracteata compared with under P. javanica in rubber plantation in India. These bacteria included N fixing bacteria and phosphate solubilising microorganisms. Hence, M. bracteata improves soil fertility.

M. bracteata produces large amounts of organic matter through its litter mulch. It becomes an extra source of manure for oil palm.
Planting methods
The total number of participants for this seminar was about 200 which was more than the expected number. They included planters from Indonesia and East Malaysia. We did not expect that some of them are from estate top management.
According to a participant from Sabah, the rooting depth of M. bracteata is 9 feet and thus it is drought tolerance. After the gardener frequently mowed M. bracteata that was planted at the backyard of his house by using a lawnmower, new leaves grew again after some time. Although the survival rate of M. bracteata is good, it still can be killed by herbicides.
A participant who has been selling M. bracteata seeds for many years and with full of experience in planting M. bracteata mentioned that seed handling method for M. bracteata is different. It should not be soaked in water in order to enhance the germination rate. If the seed is soaked in water before germination, the germination rate will drop to 10%. The soil medium that is used in the nursery should be sandy loam soil to avoid stagnant water. After sowing, he suggested to put up a plastic cover with the main intention of controlling rainwater. Watering is required once daily.

M. bracteata that was planted in FELDA nursery. These seedlings can be transplanted to the field at any time.
The quantity of seeds to purchase depends on the number of seedlings of M. bracteata to be planted within one hectare of oil palm. According to FELDA, the M. bracteata seedlings were planted in the interrows at 4 m apart giving a density of 300 seedlings per ha, reaching full ground coverage after nine months. We planted two seedlings in the interrows at 8 m apart in Indonesia. In fact, every estate has their own planting density, between 130 seedlings per hectare and 680 seedlings per ha (5 seedlings of M. bracteata per palm).
Rhizobium bacteria provide nitrogen indirectly
One of the characteristics of legume is that the root nodules are infected by rhizobium bacteria. It is able to fix N from the atmosphere and provide nitrogen to the plant indirectly.
There are many strains of rhizobium bacteria. Which strains is the most suitable for research on M. bracteata was acknowledged in the seminar. The experiment was conducted in Universiti Putra Malaysia to screen Bradyrhizobium strains that can effectively nodulate M. bracteata . FiveBradyrhizobium strains were tested for effectiveness as legume inocula. From the experiment, M. bracteata inoculated with isolate UPMR51 achieved the highest growth rate and produced the largest nodule number with the greatest N concentration.
AAR showed that M. bracteata can fix 70% of nitrogen for its own consumption. From the analysis and calculation, absorbing one unit of nitrogen is equivalent to releasing two units of nitrogen to the soil.
Rhizobium bacteria provides nitrogen to M. bracteata and the rich biomass of M. bracteata produces green manure. Can young palm rely on M. bracteata to provide all or most of the nitrogen? Agronomists have been doing research on this.
Some smallholders in Indonesia cannot afford to buy chemical fertilizers resulting in decreasing oil palm yields. We helped to plant M. bracteata in these poor oil palm gardens in order to know how much of the cost of chemical fertilizers we could save for smallholders by planting M. bracteata.
From the experiment in EPA oil palm plantations in Johor, after planting for two years, M. bracteata dry vine weight was 5.7 tonne per ha, root and root nodules were 2.5 tonne, stem and leaf were 3.0 tonne, total biomass was 11.02 tonne. Excluding leaf litter, the total biomass contained P(19kg), K(153 kg), Mg(18 kg). According to the current price of fertilizer, the biomass is equivalent to RM 1066.
Less plant disease problem
According to the Golden Hope research report, the dry weight of M. bracteata was 17.2 tonne per ha while the conventional leguminous cover crop only 5.7 tonne per ha. But, the age of M. bracteata and the soil types were not reported. By using basaltic soil, dry matter production is up to 19 tonne (11 tonne from vine, 8 tonne from leaf litter).
M. bracteata has less plant disease problem. This may due to its high concentration of phenol, thus it is able to avoid being attack by other creatures. However, young plants (less than 6 months old) are palatable to cattle but not goats.
If we walk in between M. bracteata , the fluid from the broken vines may leave some stubborn stain on our pants. It is very difficult to remove the stubborn stain and the pant may have to be thrown away.
The seminar was held successfully. We should carry on our effort. In my opinion, I felt that the title of the next seminar may be “Analysis of oil palm planting materials”. FELDA is an ideal place to hold this seminar because FELDA produced 17,300,000 oil palm seeds in 2006, 22,000,000 oil palm seeds in 2005, 23,000,000 oil palm seeds in 2004, and has 26.3% market share. If smallholders have a chance to take part in the seminar (English medium), they can know more about the process of seed production and it helps in choosing good planting materials.

At the moment, M. bracteata is already well known in the plantation industry. It has proven to be an useful cover crop.
Precision Farming: Concluding Remarks
[addw2p name=”precisionFarming”]
Precision farming is goal oriented and hinges on the advent of information technology, affordability of key technologies such as GPS, sensors and yield monitors, agronomic knowledge of the crop and management strategy and system. Its applicability for oil palm plantations also depends on the success of mechanisation of most field operations e.g. fertilisation, harvesting and collection, and the attitude and readiness of management to change.
Precision farming is a cropping system and therefore, we should not be too preoccupied with the technology but to listen to the management’s needs and devise, improve and match resources and agricultural practices with the soil and crop requirements for efficiency and high productivity. Precision farming also requires better and more progressive managers to implement and ensure its success.
Precision Farming: Future Work on Precision Farming for Oil Palm Plantations
[addw2p name=”precisionFarming”]
Precision farming has not been implemented in the oil palm plantations to the best of our knowledge although the tools and technologies associated with it have been widely utilised. The major obstacles to precision farming in oil palm plantations are probably capital expenditure, lack of quantification of cost-benefit and risk of the new practices and resistant to change, which can be expected at this early stage of development. Currently most of the research is on adapting the new tools and technologies to solve immediate problems with little thoughts given to strategic and tactical approaches. As pointed out by McBratney and Taylor (1999), “To make precision farming work all areas of the Precision Agriculture wheel (Figure 3) need to be addressed”.
We do not intend to provide a comprehensive account of the future work required to introduce precision farming successfully to the oil palm plantations as it is beyond us. Instead, it should suffice to list out some important areas where further research may be warranted.
Mechanisation of most operations (Figure 15) is an absolute necessity for precision farming to materialise in oil palm plantations. The machines will allow dataloggers, yield monitors, GPS, sensors etc to be fitted for data collection. However, the errors and precision of the data and generated yield maps need further investigation.
Figure 15: Examples of mechanisation of field operations in oil palm plantations

Differential GPS in the market are sufficiently accurate for geo-referencing in horizontal direction (< 5m accuracy is sufficient for oil palm plantations) but inadequate for vertical direction (currently accurate to 1-3m) and generation of good DEM for assessing among other, drainage requirement, terracing and re-direction of water into the fields (rain harvest and watershed management).
Agonomic research should be targeted on understanding yield variation at a finer scale than currently done. The results are important towards the success of attribute mapping and interpretation of yield maps, and development of decision support system (DSS). The former is confounded by the perennial nature of oil palm which exhibits strong temporal yield variation and 2 to 5 year yield cycle. Methods to combine spatial and temporal data (Blackmore, 2000) and a protocol to interpret yield maps (e.g. Larscheid et al ., 1997) should be develop for oil palm. Decision support system translates data into knowledge for differential actions in the fields generally by combining database, crop models, expert system and artificial intelligence. Work should commence on the next generation of DSS which will be web-based and accessible through hand-held equipment including a telephone.
Variable rate technology (VRT) is an engineering problem (McBratney and Taylor, 1999) but much research is still needed to make it work in oil palm plantations.
There is much scope for management improvement in precision farming particularly in increasing labour productivity via better infrastructure, more precise planning, organisation, budgeting and worker friendly practices (Chew, 1998). Ultimately, it is the management who will make precision farming in oil palm plantations a success or failure.
Precision Farming: Applicability of Precision Farming for Oil Palm Plantations
[addw2p name=”precisionFarming”]
The tools and technologies associated with precision farming have attracted the interest of researchers in the oil palm industry in Malaysia as illustrated by a number of recent papers such as Tey et al . (2000) on GIS, GPS and remote sensing, Goh et al. (1997) and Guha and Guha (1997) on decision support system, McMorrow et al. (2000) on remote sensing, Goh et al. (2000) on spatial FFB yield variation and Kok et al.(2000) on site-specific agronomic management. These tools and technologies provide an opportunity to understand and capitalise on the variabilities in the fields which have long been recognised by the planters but little can be done until now.
The existence of variabilities in oil palm plantations
Precision farming is only applicable if manageable variabilities exist in the fields. Two types of variability are of interest, namely, FFB yield which is the single most important factor influencing profit (Ong, 2000; Goh and Chew, 2000) and soil fertility which affects fertiliser input, the largest cost item.
a) FFB yields
The inherent palm to palm variability of FFB yield of oil palm has been studied via uniformity trials since the 1920s when it was first grown commercially. Its coefficient of variation (CV) could exceed 30% (Webster, 1938; Chapas, 1961; Goh and Alwi, 1988; Soh et al. , 1989). These CVs also vary from site to site and temporally (Goh and Alwi, 1988; Soh et al. , 1989). Part of the FFB yield variability could be attributed to soil heterogeneity where the Fairfield Smith’s heterogeneity index, “b”, commonly ranges from 0.25 to 0.93 (Goh and Alwi, 1988). Thus, Planters have demarcated their land into smaller management units or zones of 10 to 100 ha for more uniform fields and ease of implementing the agro-management practices.
However, the CV is non-spatial and does not distinguish between autocorrelated yield variation (which is manageable), and uncorrelated (‘nugget’) variation (which is not manageable) (McBratney et al. , 2000). This has prompted Goh et al. (2000b) to use geostatistical method to study the FFB yield variation in a fertiliser response trial of about 25 ha where FFB yields of individual palms were recorded. Results showed that the mean random (nugget) variation accounted for only 26% of the total variation (Table 2). About 74% of the FFB yield variation could be managed spatially if its causative factors are known.
Table 2: Semivariance analysis (standardised) of FFB yields of oil palm without N and K fertilisers from 1991 to 1998
| Parameter |
1991 |
1992 |
1993 |
1994 |
1995 |
1996 |
1997 |
1998 |
Mean |
| Total |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
| Nugget |
0.23 |
0.28 |
0.28 |
0.28 |
0.22 |
0.27 |
0.28 |
0.22 |
0.26 |
| Spatial |
0.77 |
0.72 |
0.72 |
0.72 |
0.78 |
0.73 |
0.72 |
0.72 |
0.74 |
| Range (m) |
15 |
21 |
12 |
18 |
15 |
9 |
15 |
21 |
16 |
After Goh et al . (2000b)
b) Soils
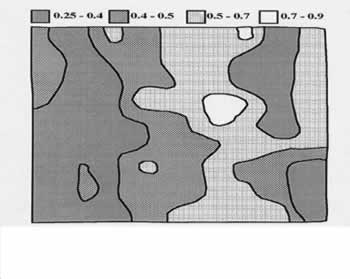
Malaysian soils have generally low soil fertility and hence, large responses to fertilisers are commonly obtained in the oil palm plantations (Gohet al ., 1999). Apart from this, the soil nutrient contents vary considerably (CVs commonly exceed 40%) even within the same soil series as shown by Ng and Ratnasingam (1970) and Law and Tan (1977) for Peninsular Malaysia and Goh et al. (1998) for Sabah. Ng and Ratnasingam (1970) further showed large variations and spatial patterns of nutrient contents for individual soil types e.g. exchangeable K distribution (Figure 4) in a 11 ha field of Selangor series soil, which was derived from marine alluvium and considered to be fairly homogenous by soil profile examination (Chew, 1998). Their results indicated that two-third of the field would not require K fertiliser while the balance, mainly in the western halve, would need low K fertiliser rate to sustain growth and production.
Figure 4: Distribution of exchangeable K values (cmol(+)/kg soil) in 11 ha oil palm field of Selangor series soil (source: Ng and Ratnasingam, 1970)
Soil variations could also arise from previous planting practices such as fertiliser application areas, frond placement and harvesters path (Kee et al. , 1995; Goh et al ., 1996). Goh et al . (1996) also found high spatial variability within micro-sites of palm circles, frond piles and interrows within single palm areas from previous fertiliser application practices with resultant nutrient patches within 2 m of each other (Table 3). There are many other known sources of soil variation such as micro-relief and these will not be discussed in the paper.
Table 3: Score of nutrient patches around individual oil palms on Musang series (Typic Paleudult) soil
|
Soil depth |
Site |
Fertiliser |
|
|
Without |
With |
||
|
0-15 |
Palm circle |
8 |
12 |
|
Interrow |
8 |
6 |
|
|
Frond heap |
15 |
12 |
|
|
15-30 |
Palm circle |
12 |
12 |
|
Interrow |
7 |
6 |
|
|
Frond heap |
11 |
12 |
|
Note: Maximum score of 15 for best soil fertility and minimum score of 5 for poorest soil fertility
Source: Goh et al . (1996)
Tee (Unpublished) has studied the nature of soil NH4+ -N and NO3– -N in a long-term fertiliser response trial in Sabah, Malaysia where N fertiliser was applied in the palm circle (within 2 m radius of the palm). She found that the inherent soil NH4+ -N and NO3– -N i.e. in areas without nitrogen application for the past 10 years had higher CV compared to manured areas (Table 4). However, the CVs in the latter sites were still large with 45% for NH4+ -N and 81% for NO3– -N. But surprisingly almost all the variations were spatially related (Table 5) and therefore, manageable. The spatial ranges for NH4 + -N and NO3– -N were also substantially reduced with manuring. Interestingly, the spatial range for NH4+ -N in manured areas was 51.4 m, which was equivalent to 5 to 6 palm distance and corresponded well to the experimental plot size. Further work is necessary to ascertain and understand the results.
Table 4: Mean and variation of soil ammonium- and nitrate-N in the palm circle of oil palm on Kumansi Family soil in Malaysia
| Nitrogen | Nutrient |
Mean (mg/kg) |
CV (%) |
| Without |
NH 4 -N |
27.2 |
54.9 |
| With |
NH 4 -N |
140.9 |
44.8 |
| Without |
NO 3 -N |
7.5 |
102.5 |
| With |
NO 3 -N |
9.7 |
80.5 |
Note: CV denotes coefficient of variation
After Tee (Unpublished)
Table 5: Nature of variations of soil ammonium- and nitrate-N in the palm circle of oil palm on Kumansi Family soil in Malaysia
| Nitrogen | Nutrient |
Total Variation |
Random |
Spatial |
Range (m) |
| Without |
NH 4 -N |
2.44 |
0.18 |
2.26 |
254.3 |
| With |
NH 4 -N |
1.06 |
0.09 |
0.97 |
51.4 |
| Without |
NO 3 -N |
1.23 |
0 |
1.23 |
71.6 |
| With |
NO 3 -N |
1.02 |
0.06 |
0.96 |
19 |
After Tee (Unpublished)
Maximising FFB yields
The major factors affecting oil palm yield are known and their effects have been quantified by various researchers such as Foster et al . (1985) and Kee et al .( 1994). Goh et al. (2000a) presented an outline of an empirical model called ASYP, which has been validated (Kee et al ., 1994; Kee et al ., 1999) and used commercially as shown in Figure 5. The model predicted that site yield potential of a field is more or less predetermined after planting. Very little can be done to change the variable factors such as planting density and pattern till replanting more than 20 years later (Chew, 1998). Uneven planting with over-crowded spacing or high vacancies particularly in hilly, rugged terrain (Figure 6) can result in poor uniformity of growth, light utilisation and exploitation of soil nutrients and water for maximum production. This common problem provides an excellent opportunity for precision farming by making full use of remote sensing and digital elevation model (DEM) to demarcate the terrain as shown in Figure 7 and coupled with soil maps for specific practices such as higher planting density for peat soil (160 palms per ha) and shallower soils (148 palms per ha). We can also automatically generate precise positions of the terraces for constant density planting in hilly areas as illustrated in Figure 8 (Tey et al ., 2000). The latter has always been a Planter’s dream but could be a reality now with the new tools and technologies.
Figure 5: Primary factors influencing the site yield potential of oil palm in Malaysia

Source: Goh et al. (2000a)
Figure 6: Ikonos remote sensing image (1 m resolution) shows areas with large vacancies and uneven plantings.
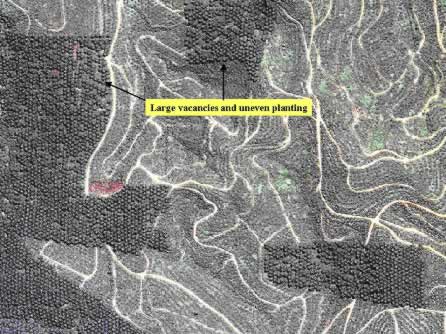
Source: Space Imaging Inc., Singapore.
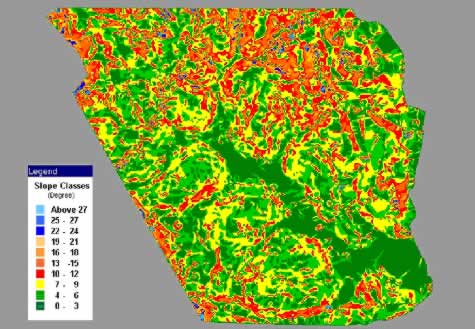
Figure 7: A 5 meter-pixel slope map derived from the DEM of the study area allows the demarcation of field by terrain.
Source: Tey et al . (2000)
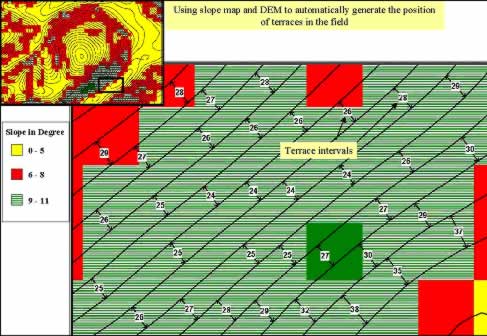
Figure 8: Using DEM and slope map to automatically generate the proposed terraces for constant density planting in oil palm plantation
Source: Tey et al . (2000)
The soil factors in the production equation of oil palm (Figure 5) could be easily determined by detailed soil survey (Goh et al ., 1997). This traditional process is essential for maximising FFB yields because the different marginal and problem soils in the oil palm plantations would require separate management strategies and agricultural practices (Goh and Chew, 1995) as shown in Figure 9. Of more importance is perhaps the identification of specific, main soil and agronomic limiting factors (e.g. shallow, lateritic soils) and reclassification of common soils in each field into practical management zones that are bordered by roads and precisely located in GPS maps for ease of implementing the prescribed remedial actions (Figure 9). The impact of site-specific management was demonstrated by Goh et al. (1997) in a semi-commercial trial where each field was separated into lateritic and non-lateritic areas in 1986 (Table 6). The overall FFB yield of the 216-ha area increased from 18.4 t/ha/yr in the period of 1984-86 where uniform management was practised to 26.3 t/ha/yr in 1990-92 after implementation of site-specific inputs from 1986 (Kok et al ., 2000). At palm oil price of RM 1000/tonne, profit per hectare was 35% better in the period after site-specific management (Goh et al ., 1997).
Figure 9: Creation of practical management zones for site-specific agricultural practices 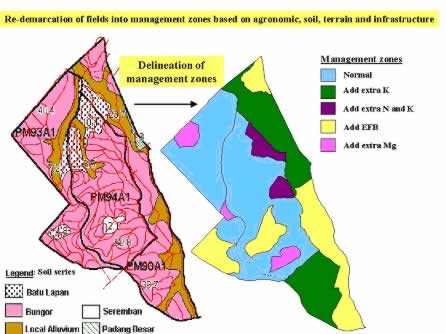
Adapted from Kok et al. (2000)
Table 6: Effect of improved agromanagement inputs and site-specific management on FFB yields
|
Details of site |
FFB yield (t/ha/yr) |
||||
| Field | Soil type |
Site yield potential (t/ha/yr) |
Period 1
|
Period 2 |
Period 3 |
| PM81A | Non-lateritic |
32.6 |
19.6 |
30.1 |
32.4 |
| PM81B | Non-lateritic |
28.6 |
15.8 |
26.7 |
28.0 |
| PM82 | Non-lateritic |
30.1 |
17.3 |
33.0 |
32.8 |
| PM77 | Non-lateritic |
30.2 |
19.3 |
29.3 |
28.0 |
| PM72 | Non-lateritic |
27.0 |
19.0 |
25.0 |
25.0 |
| Weighted mean |
28.6 |
18.4 |
27.9 |
27.6 |
|
| PM81A | Lateritic |
26.8 |
19.6 |
25.1 |
30.6 |
| PM81B | Lateritic |
23.4 |
15.8 |
22.5 |
25.8 |
| PM82 | Lateritic |
26.7 |
17.3 |
25.2 |
28.0 |
| PM77 | Lateritic |
25.6 |
19.3 |
24.0 |
25.4 |
| PM72 | Lateritic |
23.1 |
19.0 |
21.7 |
22.9 |
| Weighted mean |
24.4 |
18.4 |
23.0 |
25.1 |
|
Note: Uniform management of each field in period 1. Site yield potential of each site was estimated using ASYP model in 1986.
After Goh et al. (1997)
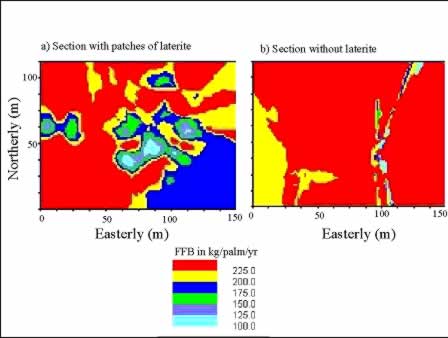
The management zones can be further delineated if FFB yield maps are available as shown in Figure 10. Even in a relatively uniform field with similar management practices, there are distinct areas of high and low yielding palms, e.g. a patch of low yielding palms in the lower right-hand corner of Figure 10a and western corner of Figure 10b.
Figure 10: FFB yield maps of oil palm in 2 different sections of a relatively uniform field
Source: Tee (Unpublished)
Quick identification of problems and nipping them in the buds are key steps towards maximising FFB yields in the plantations. Two examples are provided in the paper. Firstly, we have used GIS and GPS to improve drainage in an estate (Tey and Chew, 1997). This problem requires an overview of the watershed on a regional basis which is commonly beyond the estate boundary and covers land owners. With the new tools, we can easily compute the volume of excess water to drain, determine the direction of main drain, design a system of collection and field drains and compartmentalise areas for specific drainage design (Figure 11).
Figure 11: Design of drainage system in an oil palm plantation using GIS, GPS and DEM
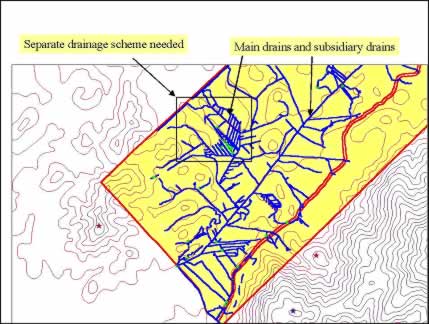
Adapted from Tey and Chew (1997)
Another example is the assessment of the extent and progress of pest damage in an oil palm plantation using remote sensing images (Figure 12) where the ability to survey affected areas quickly is a big advantage in pest and disease management (Chew, 1998). We can also identify the direction of the pest movement, which is easterly in the example, and the focal points where the next outbreak is likely to occur. This allows for differential treatments and timing of treatments to contain and ultimately eradicate the pest.
Figure 12: Quick assessment of pest damage and movement using remote sensing image
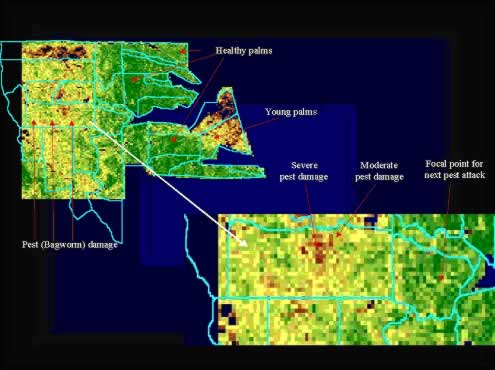
Adapted from McMorrow and Tey (2000)
Optimising inputs through precise actions
a) Management zone
The current technologies, information and management level at the oil palm plantation do not allow the precise management of single oil palm. Therefore, creation of management zones within the plantation based on palm age, agronomic and soil information and infrastructure is still the most effective means to optimise inputs as discussed earlier. The difficulty here is to decide on the scale or size of each management zone, which is probably too big now at 10 to 100 ha. Early work shows that the spatial variation of FFB yield is isotropic with a range of about 3 palm distance (Goh et al ., 2000b). This means that the optimum size of a management zone is 32 palms given the triangular spacing in oil palm planting. But with the common road spacing of 20 palm distance, the minimum, practical management size should be 140 palms (7 palm rows x 20 palms per row) or about 1 ha. Further work is needed to ascertain this.
b) Fertilisation
The principal agronomic constraint to high productivity is usually inadequate soil nutrient supply (Chew, 1998), which is corrected by large amount of fertilisers. Thus, fertiliser is the largest cost item in the production of oil palm in Malaysia. It constitutes about 60 – 70% of the field upkeep cost of oil palm. Chew (1998) further contended that “Wrong fertilisation techniques may result in high financial losses through loss of crop or excessive fertilisation and risks of high nutrient losses in run-off, leaching and other nutrient loss mechanisms”. Precision farming appears to offer some solutions to the problem.
Classical fertiliser response trials on different soil types showed highly variable FFB yield responses to N, P and K fertilisers, ranging from 0 to over 100 %. These variations could be partially reduced with management zoning of the fields and correct fertilisation (Goh et al. , 1999). However, the real opportunity to optimise fertiliser inputs lies in the understanding of the large variation in fertiliser responses within the same soil series and similar terrain as shown in Table 7. In a first attempt, we have used the plots with and without nitrogen applications in a classical fertiliser response trial (about 25 ha) as sampling points for generation of yield maps by kriging. Results showed that the yield response to nitrogen varied spatially across the trial site (Figure 13). They ranged from good FFB yield response of more than 50 kg/palm/yr in the central portion of the field to poor or negative response in the eastern and western parts. These results might be partially explained by the localised high soil N contents and the presence of a thin layer of laterites in the central portion which hinders root development leading to higher N response. This study indicates that differential N input is probably more cost effective and environment friendly.
Table 7: FFB yield responses to 1 kg Ammonium sulphate (AS) applied per palm per year and corresponding yield levels at non-limiting rates of all other fertilisers in trials on some sedentary soils in Malaysia
| Soil series |
N1 response (t/ha/yr FFB) |
N1 yield |
N2 response (t/ha/yr FFB) |
N2 yield |
| Batu Anam |
0.53 |
28.06 |
0.68 |
29.57 |
| Batu Anam |
1.46 |
22.39 |
1.78 |
26.41 |
| Batu Anam |
2.56 |
18.14 |
1.24 |
22.85 |
| Batu Anam |
2.40 |
17.53 |
1.49 |
21.44 |
| Munchong |
1.33 |
23.70 |
0.47 |
24.60 |
| Munchong |
1.02 |
30.40 |
0.46 |
32.13 |
| Munchong |
-0.65 |
27.59 |
-0.75 |
25.40 |
| Munchong |
0.54 |
25.95 |
0.43 |
27.09 |
| Rengam |
1.38 |
25.03 |
0.72 |
27.39 |
| Rengam |
3.46 |
23.81 |
1.22 |
29.08 |
| Rengam |
-0.88 |
23.04 |
-1.35 |
22.28 |
| Rengam |
1.92 |
24.31 |
0.98 |
29.59 |
| Rengam |
0.78 |
26.49 |
0.56 |
27.70 |
| Rengam |
0.82 |
27.19 |
0.64 |
28.50 |
Source: Chew (1998) who adapted it from Foster et al . (1985)
Figure 13: Spatial FFB yield response of oil palm on Kumansi Family soil to N fertilisers
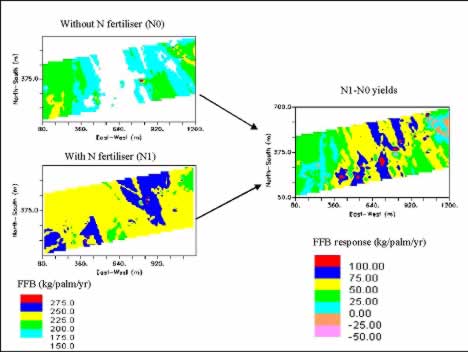
Apart from getting the fertiliser rates right and applying them in the correct places, timing of fertiliser applications holds the most promise for improving efficiency. This is because run-off and soil erosion are the most important pathways for soil and fertiliser nutrient losses. Both processes are driven by rainfall which is difficult to predict in the tropics. However, using expert system and artificial intelligience we have developed a package which predicts the best months to apply fertilisers taking into account the fertiliser properties, agronomic data such as palm age and nutritional status, soil data such as terrain and consistency, management resources, risk management and weather. The present management system including the size of fertiliser store generally does not allow the applications of different fertilisers in different fields in the same month or to apply the fertilisers for each field in the few best months available (problem with labour resource allocation) unless fertiliser applications are fully mechanised. Hence, the program also compares the predicted best months to apply fertilisers with the actual recommended months of applications to determine the potential loss in fertiliser efficiency (Table 8) which can be corrected with higher fertiliser rates, albeit at a “financial loss”.
Table 8: Scheduling of fertilisers for the oil palm plantations using expert system
| Manuring Block | Month/yr | Fertiliser type |
Rate (kg/palm) |
Suitability of month | Remarks |
| 1 PR97A 2 | Jul-98 | AS |
0.75 |
Good | – |
| Aug-98 | AA2 |
1.00 |
Fair | Maximum rate | |
| Sep-98 | KS |
1.25 |
Poor | – | |
| Nov-98 | AA2 |
1.00 |
Poor | Maximum rate | |
| Dec-98 | JRP |
3.50 |
Poor | – | |
| Jun-99 | MOP |
1.25 |
Good | – | |
| 1 PR97A 3 | Jul-98 | AS |
0.75 |
Good | – |
| Aug-98 | AA2 |
1.00 |
Fair | Maximum rate | |
| Sep-98 | JRP |
3.50 |
Poor | – | |
| Nov-98 | AA2 |
1.00 |
Poor | Maximum rate | |
| Jun-99 | MOP |
1.25 |
Good | – |
c) Infrastructure
In a good oil palm plantation of 2000 ha, there are approximately 200 km of roads to transport about 50000 tonne of FFB to the mill each year. DEM coupled with decision theory such as shortest path and network with constraints e.g. setting maximum slope of the road at 10% can be used to re-design the road system for more efficient transport of FFB to the mill as shown in Figure 14 (Tey, Unpublished).
Figure 14: Redesigning road system in an oil palm plantation using DEM for efficient transport of FFB, workers etc
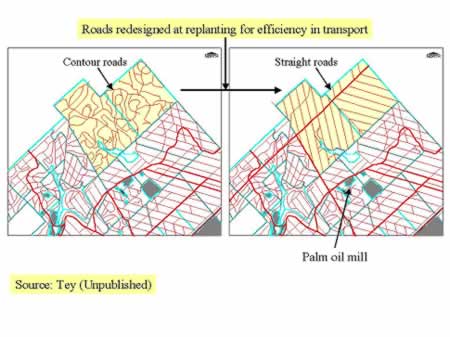
Identify areas for replanting and planting
Emmott et al . (1997) stated that “If replanting is a key issue in plantation crops, then identifying where yield improvements can be achieved in order to realise a satisfactory return on investment might be appropriate area for precision farming”. This is probably true in Peninsular Malaysia where the oil palm is getting older and probably past its prime productivity due to management difficulty in maintaining sufficient leaf area index (Goh and Chew, 2000), harvesting standards and collection of loose fruits. The potential yield loss could be examined by comparing the actual yield obtained from the field against its site yield potential, which can be estimated using ASYP model as discussed earlier. The yield gaps may then be classified and displayed on GPS maps to form a basis for strategic decision (trade-off) on replanting program of the company rather than to use an arbitrary yield level such as below 18 t/ha/yr which varies with the palm oil price or at palm age of say 25 years old (Goh et al ., 2000).
Similar approach can be taken to decide on planting or land conversion to oil palm and avoid unsuitable areas such as steep hills and swamps (Goh et al ., 1997).
Monitoring and assessment of results
One of the cornerstones of precision farming is to precisely monitor and assess the agricultural enterprise at a local and farm level (Blackmore, 1994). The oil palm industry has religiously collected agronomic and management data at the field level where possible. However, to quote Gohet al . (1999) “Of late, there is a discernible move towards larger manuring blocks in the estates with many of them exceeding 100 ha. The main reasons for this are unknown although the undertone is that management will be easier especially for large estates. Such practice, which is a form of sweeping generalisation, is definitely wrong and will make a mockery out of fertiliser management. It can also easily negate the huge investments in cost, time, manpower and equipment in the preparation of precise fertiliser recommendations”. Similarly, yield data are being combined from many fields rendering them almost useless for assessment purposes. This need not happen if we use remote sensing, GIS, GPS and electronic gadgets such as Palm organisers to collect data digitally at the estate level and adapt information technology such as decision support system to store and collate data and report the results at appropriate scales for the management.
Accurate and precise maps and area of each field are essential because most productivity figures for assessment of results and performances are based on per area (hectare) basis. In the absence of expensive land surveys, GPS and GIS mapping can provide precise field sizes apart from road, soil and terrain maps (Chew, 1998). Chew (1998) also reported that errors in hectareage commonly exceeded 10% in the plantations (Table 9).
Table 9: Differences in declared hectareages in individual blocks in oil palm plantations after GPS mapping
| Estate | Area (Ha) |
Total number of blocks |
Ha difference (%) |
||||
|
< 2 |
2-5 |
6-9 |
10-15 |
>15 |
|||
| 1 |
639 |
7 |
1 |
– |
– |
5 |
1 |
| 2 |
864 |
38 |
1 |
2 |
6 |
5 |
24 |
| 3 |
974 |
16 |
3 |
7 |
3 |
2 |
1 |
| 4 |
811 |
27 |
3 |
3 |
4 |
9 |
8 |
Source: Chew (1998)
Yields in the plantations are usually estimated from bunch count numbers and average bunch weight for the fields. The number of bunches from each task (usually 1-2 ha) or platform can be recorded using a Palm organiser such as PalmPilot and electronically transferred to the database to compute harvester and field productivity quickly. Yield maps can also be generated for the purposes discussed above. With in-field mechanisation and collection of FFB, the prospects for more precise yield monitoring at finer scale are good (Chew, 1998) and should be encouraged.

