Rubber: Pests and Diseases
Control of White Root Disease in Immature rubber with 3 systemic fungicides.



Contributor : Chan Weng Hoong
Rubber: Manuring
Manuring in rubber: need for re-evaluation based on case study.

Contributor : Chan Weng Hoong
Rubber: Selection of Clones
Performance of Clone PB260 in a large plantation group in Peninsular Malaysia.

Contributor : Chan Weng Hoong
Rubber: Exploitation
AAR jacket system: A Promising Improved System of extracting latex from rubber trees.

Evaluation of three types of rainguards.

Use of Short Cuts to extend Lifespan of PB 260.
 .
.
Contributor : Chan Weng Hoong
Rubber: Rubber Outlook
|
Interest in rubber has recently resurfaced due to the increase in price of the commodity. Price of rubber has risen from RM3.50 per kg in 2002 to RM8.00 in 2006. Rubber price has been predicted to remain at current equitable prices for a while due to the following :
In view of the above, the Company has considered returning to rubber where terrain is steep and rainfall low where oil palm has not yielded well. |
 |
|
Past and current research interests are :
|
|
|
|
|
|
Contributor : Chan Weng Hoong |
|
Nutrition: Conclusions
[addw2p name=”oilPalmNutrition”]
The annual B requirement of oil palm increases rapidly and reaches a peak at six years after planting. B is mainly needed for the canopy development and fresh fruit bunch production. Majority of the absorbed B is phloem immobile but there is an indication that B in the petiole and rachis of old leaf is phloem mobile. By the time of replanting, B immobilized in the stem is sufficient to meet B requirement of oil palm in its first four years of growth and production. A management strategy is therefore required to recycle this substantial amount of B to the next generation of oil palm.
Nutrition: Discussion
[addw2p name=”oilPalmNutrition”]
The B requirement of oil palm in the first 82 months after planting was mainly driven by its growth rate, a process termed as growth demand for nutrients by Tinker and Nye (2000). This is due to the relatively constant B concentration across palm age in each palm component, which is in agreement with the results of Ng et al. (1968). However, the current Tenera oil palm on infertile Oxisols requires about 27 % more B than the older Dura planting material on fertile Inceptisols. This difference might be attributed to the larger canopy of the Tenera oil palm which is responsive to high fertilizer regime (Goh et al ., 2003).
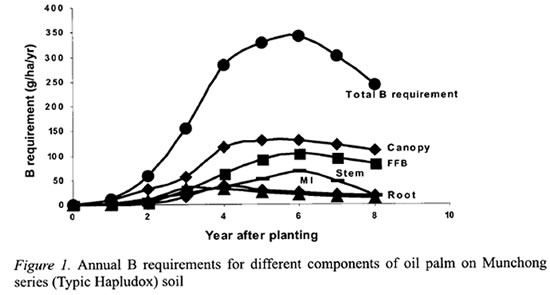
The B content of the roots had not been studied in the oil palm. Initially it partitions a large proportion of its biomass to the roots to maximize the exploitation of soil water and nutrients. This results in relatively large B requirement for root development. But as the other vegetative matter develop and production commences, the proportion of B for roots declines to less than 6 % (Figure 1).
The annual B requirement decreased from the sixth year after planting due mainly to the declining growth rate of the stem (Figure 1). Extrapolating the model for the annual B requirement shows that the steady-state B uptake of 198 g ha-1 yr-1 as estimated using the 16 years old palms in the second experiment would be reached at the twelfth year after planting. This corresponds with the findings of Gerritsma and Soebagyo (1999). They found that the vegetative growth of oil palm reached its maximum at the twelfth year after planting, and FFB yields were relatively stable thereafter. Therefore, the nutrient requirements including B can be anticipated to be in a steady state too.
The Tenera fresh fruit bunches contained 2.39 mg B kg-1 which was only 12 % greater than Dura bunches reported by Ng et al. (1968). This was despite its bigger mesocarp and thinner shell and the former containing more B. This lack of difference could be partially attributed to the higher B concentrations in the stalk and empty spikelets of Dura bunches although strong conclusion could not be made since Ng et al . (1968) analyzed only two bunches compared with 26 bunches in this study.
The B distribution in the vegetative components and FFB of oil palm suggests that B is mainly transported by the xylem. In the oil palm, B is considered to be phloem immobile based on the work of Rajaratnam (1972). Moreover, B toxicity symptoms of oil palm are exhibited first in the tips and margins of leaflets of older leaves suggesting that it is a non-polyol producing plant (Brown et al ., 1999) and therefore, its B has restricted phloem mobility. However, a detailed analysis of the B concentrations in the canopy implies that B may be translocated in the phloem also. The young developing (spear) leaves had B concentration similar to the matured leaves (leaves 9 to 16) and higher than the maturing leaves (leaves 1 to 8). But the older leaves (leaf 33 and older) tended to have the lowest B concentration (Table 5). The declines in B concentration occurred in the petiole and rachis but not the leaflets (Table 6). This indicates that B may be mobile in the phloem of petiole and rachis only. Both Ng et al . (1968) and Rajaratnam (1972) did not investigate the B concentrations in the spear leaves and leaves older than 33. Thus, they did not observe the above and the latter concluded that B was phloem immobile while the former contended that there was no consistent trend. Rajaratnam (1972) further illustrated that B could be lost from the leaves through guttation in order to explain the differences in B contents in the leaves at different time of sampling and leaf age. However, this postulation could not explain our results since guttation can occur in all the leaves.
The oil palm is mainly planted on highly weathered Ultisols and Oxisols with generally low soil B content. Thus, increasing rates of soluble B fertilizer in the first six years after planting are usually required to match the annual B requirements (Figure 1). Apart from the first year, the B rates should range between 2.25 and 4.5 kg B ha-1 yr-1 due to the low fertilizer use efficiency of less than 15 % as obtained in this study. Subsequently, the B rates may be decreased to between 2 and 3 kg B ha-1 yr-1 for palms between seven and twelve years old. At the steady state of 12 years or older, only occasional B applications are probably necessary since B exported out of the system through FFB and immobilized in the stem is relatively low.
About 43 % of the B in FFB is found in the stalk and empty spikelets which can be returned to the fields via empty fruit bunches. It is probably also worthwhile to build-up B in the oil palm canopy in the early years to increase the B content in the leaf pile, which is the dominant source of organic matter in the plantations. This is because organic matter can be an effective source of B by mineralization when the soil B is low (Bell et al ., 2002).
The oil palm accumulates substantial B in its vegetative components and by the time of replanting at 25 years old, it should reach about 750 g B ha-1. This is sufficient to meet the B requirements of oil palm in the first four years. The current zero-burn replanting technique where the palms are felled, chipped and pulzerized, and the organic residues spread throughout the field may be able to return the palm B to the soils although the next generation of oil palms may not be able to exploit it fully due to its initial small root system and the large leaching loss of B in Malaysian soils (Rajaratnam, 1973b).
Nutrition: Results
[addw2p name=”oilPalmNutrition”]
B requirements of the oil palm
The B contents of the canopy and stem increased rapidly from 20 to 82 month after planting (Table 1). These increases were mainly due to palm growth since their B concentrations were relatively constant over the period of measurements. Root B content was highest (44 g ha-1) at 37 months after planting but it quickly stabilized thereafter at an average of 34 g ha-1 (Table 1). The B requirements for FFB yields and production of male inflorescences between the sampling intervals increased exponentially before reaching a plateau at 71 months after planting (Table 1), again due to biomass increments rather than B concentrations in the palm components.
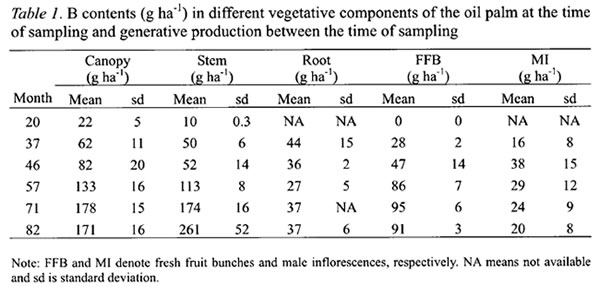
The cumulative B contents for each palm component followed a non-linear regression model (Table 2). The cumulative B contents for canopy and stem fitted well to modified exponential models whereas those for roots and FFB yields to hyperbolic models. The latter implied that the B requirements were relatively constant after their maximum has been attained. The r-squares for all the equations exceeded 0.78.
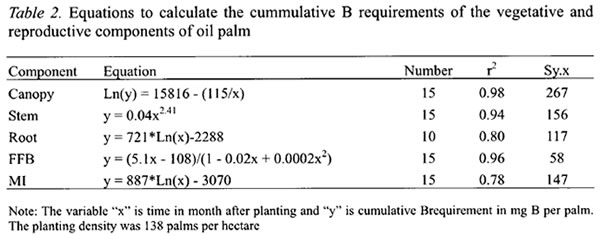
The annual B requirements of oil palm followed a sigmoidal curve with rapid increase from the second year after planting, reaching a maximum of 343 g B ha-1 yr-1 in the sixth year (Figure 1). The annual B requirement then declined to about 220 g B ha-1 yr-1 in the eighth year before stabilizing at 198 g B ha-1 yr-1 from the twelfth year. The latter was based on the estimated annual B requirement of the 16 years old palms at steady state condition in the second experiment. The highest B requirement of oil palm came from its developing canopy, which peaked at the fifth year after planting when the canopies of neighboring palms were fully overlapped (“closed”) and competition began to set in. At the peak, the B requirement for the canopy accounted for more than 40 % of the total B requirement of oil palm.
The B requirement for stem development was also the highest in the sixth year at 67 g B ha-1 yr-1 before declining sharply as palm competition intensified and growth rate slowed down (Figure 1). In contrast, the B requirement for roots was not only the lowest but also attained its peak earlier at the third year after planting (Figure 1). Thereafter, it required about 9 g B ha-1 yr-1 to sustain root turnover or self pruning of roots.
The B requirement for FFB yield was the second largest among the palm components and account for about 33 % of the annual B requirement at the sixth year and increasing to 37 % as the B needs for stem decreased sharply (Figure 1).
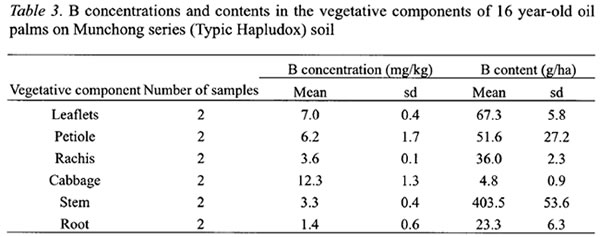
B distribution within the oil palm
The detailed B distribution in the oil palm was studied in the second experiment where the palms were 16 years old and in steady state condition (Tables 3 and 4). The cabbage, which composes mainly meristemic cells, had the highest concentration of B at 12.3 mg kg-1 (Table 3). This was followed by the leaflets which were at the tail-end of the transpiration stream. Interestingly, the petiole, which supports the rachis and leaflets, and the petiole base, which are attached to the stem after the leaf is pruned off, had higher B concentrations than the rachis or stem. The stem and rachis had similar B concentrations of 3.3 and 3.6 mg kg-1, respectively. Although the stem B concentration was low, it has accumulated a large quantity of B by the 16th year at 404 g ha-1. The roots had the lowest B concentration of 1.4 mg kg-1.
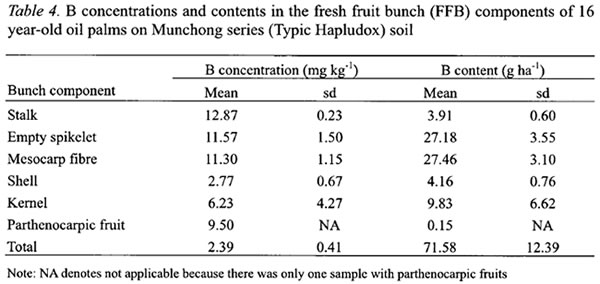
In FFB, larger B concentrations were found in the stalk, empty spikelets and mesocarp fibre (Table 4). These components have more rapid transpiration rate particularly during fruits development and oil formation stages (Jeje et al ., 1978). B concentration in the kernel at 6.23 mg kg-1was 2.25 times more than the shell (Table 4). The stalk and empty spikelets, which are the main components of empty fruit bunches after the milling process, contained about 43 % of the B in FFB.
A detailed analysis of the B distribution in the canopy showed that the B concentrations seemed to be relatively constant from the spear leaves to leaf number 32 before decreasing in the older leaves (Table 5). In terms of B content, there was an initial increase from leaf 1 to leaf 9 due to increasing frond dry weight. The B contents between leaf 9 and leaf 32 were similar at an average 26.3 g B per frond. The B contents then declined linearly between leaf 33 and leaf 48. This decrease was mainly due to lower dry weights and B concentrations of both petiole and rachis (Table 6). There was no clear trend in B concentrations in the leaflets.

B distribution in an oil palm plantation
The B distribution in the various components of one hectare of mature oil palms at stead state indicated that the largest store of B was in the stem at 404 g ha-1 followed by the canopy at 155 g ha-1 (Figure 2). The ground vegetation of mainly grasses and ferns contained very low B at only 6 g ha-1.
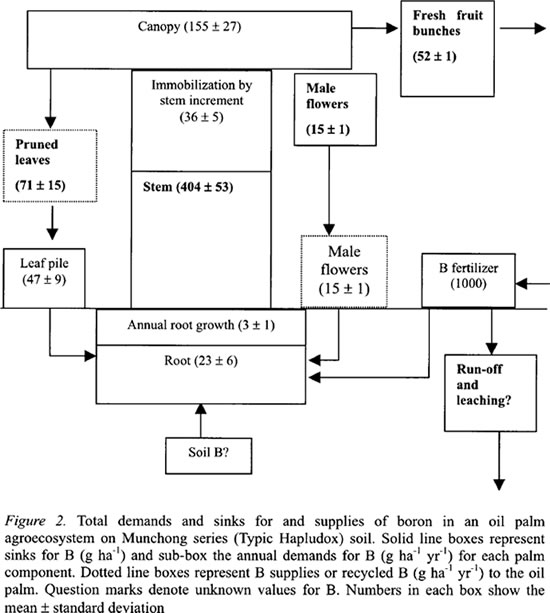
In terms of B cycling, the pruned leaves would return 71 g B ha-1 yr-1 to the agroecosystem and the decaying male inflorescences about 15 g B ha-1 yr-1. The pruned leaves were neatly stacked in leaf piles in the area between the palm rows called the inter-rows. The latter area stored about 47 kg B ha-1, which was 66 % of the annual B in the pruned leaves. The lower B value in the leaf pile might be attributed to the high decomposition rate of oil palm leaf where most of it would decay within six months.
In the oil palm agroecosystem, the FFB yields are transported to the oil palm mill for processing, and this component contained 52 g B ha-1 yr-1. The oil palm also immobilized about 36 g B ha-1 yr-1 mainly for stem growth.
Nutrition: Materials and Methods
[addw2p name=”oilPalmNutrition”]
Experimental sites
The oil palms selected for study came from the optimal NPK treatments of two factorial, fertilizer response trials. Both experiments were conducted on Munchong series (Typic Hapludox) soil which was derived from shale. In the first experiment, destructive samplings of the palms were carried out at six palm ages to study the B requirements of growing palms. Two palms were sampled at 20, 37 and 71 months after field planting whereas three palms were sampled at 46, 57 and 82 months after field planting. At each sampling, the palms were chosen from different replicates and where possible, palms from the same replicates were taken across the months. Sodium borate was applied at an average rate of 3.1 kg B ha-1 yr-1.
In the second experiment, two 16 years old palms, the interrow vegetation and the frond stacks were sampled to examine the B distribution in an oil palm field at the steady state condition. Sodium borate was applied at an average rate of about 1 kg B ha-1 yr-1.
In both experiments, the number of leaves produced by each sampled palm were measured at half yearly intervals, the fresh fruit bunch (FFB) yields at 10 day intervals and the male inflorescences at quarterly intervals. These data were then summarized on an annual basis to estimate the yearly B requirements of oil palm.
Palm destructive sampling and B analysis
The oil palm can be divided into unambiguous morphological components (Tinker and Smilde, 1963). These are: leaf, which can be further separated into leaflets, leaf rachis and leaf petiole, unopened spear leaves, growing point or “cabbage”, stem, roots, male inflorescences and fresh fruit bunches. In this paper, the stem included the petiole bases, which were attached to it after pruning the leaves, and the root bole, which was the growing point for the roots.
Each fresh leaf was cut down (“fresh” being taken to mean that over half the total leaflet area was still green) and counted. Each leaf was then separated into the leaflets, petiole and rachis. The leaflets for nutrient analysis were sampled systematically by taking 1 in 10. The balance of the leaflets was then sampled for determination of fresh and dry weights. The rachis was cut into three equal sections. Each section was divided into three equal parts and a 10 cm section was cut from the middle of each part. One longitudinal half from each section was bulked for fresh and dry weight determination. Leaf petioles from leaves 1 to 6 were divided into two equal parts whereas the older ones into three equal parts. Leaf 1 was the youngest fully opened leaf. A 10 cm section was cut from the middle of each part. One longitudinal half was then taken from each section and bulked for fresh and dry weight determination. For B analysis, the leaflets, rachis and petiole were each bulked for various groups of leaves in the ratio of their dry weights. In the oil palm, each group or whorl of leaves consists of eight leaves i.e. leaves 1 to 8, 9 to 16, 17 to 24, 25 to 32, 33 to 40, 41 to 48 and 49 to 56. The unopened spear leaves were sub-sampled in the same manner as the leaf but their rachis and petiole were not separated because most of them had very short petioles.
The primary roots around the stem were cut to facilitate its felling at ground level. The cabbage was then cut off from the apex of the stem. Two 1/16th sections were sampled. The rest of the stem was divided into six equal sections. The middle 15 cm of each stem section was sampled and two 1/16th sections sub-sampled.
The roots were sampled using the trenching method. Since the palms were planted in an equilateral triangular pattern of 9.1 m and 8.9 m apart for experiment 1 and 2 respectively, the palm area was divided into three sections as follows: palm to palm of the same row, palm to palm of another row and palm to interrow area. In each section, a trench 30 cm wide and 90 cm depth was dug to the middle of the planting row or interrow area. The roots that were dug up were sieved out and collected for every 30 cm by 100 cm by 30 cm depth section and then bulked for each palm
The male infloresecences on the palms at the time of sampling were separated into immature inflorescences, mature inflorescences and old inflorescences. The FFB for B analysis were collected from the second experiment only. A total of 26 bunches were collected over a three month period. In the laboratory, each bunch was weighed, stripped and divided into its major unique components of fruit, stalk, spikelets and parthenocarpic fruits. The fruit was further separated into the shell and kernel. Fresh weight of each component was taken. Two 100 g samples of each of the chopped and ground stalk and spikelets were collected for fresh and dry weight determination. The oil in the fruits were extracted using hexane following the modified Blaak’s method (Rao et al ., 1983).
The aerial parts of the interrow vegetation in each palm area were cut and weighed. The vegetation on the palm stem was also sampled, weighed and bulked with the interrow vegetation. Only one eighth of the fresh weight was taken for analysis.
All sub-samples of the vegetative and reproductive parts were sent to the laboratory for fresh and dry weight determination and B analysis. The latter followed the Azomethine-H method (John et al ., 1975). Briefly, 1 g of plant sample was dry ashed at 530 ° C and digested with 10 ml of 1.4 M H2 SO4. The solution was then filtered through Whatman No. 1 paper. 0.5 ml of 0.05 M EDTA, 1 ml of 0.5 M ammonium acetate and 1 ml of Azomethine-H solution were than added to 1 ml of the filtrate to prevent interferences and developed the colour. The B concentration in the filtrate was then read using a UV/VIS spectrophotometer.
Computing the annual B requirements from the data
In the first experiment, the time intervals were not fixed. Therefore, the cumulative B accumulation or uptake by the palm in each component at each sampling time has to be computed and the results modeled using non-linear regression. The gradient of the model at each yearly time period gave the annual B requirement for the component.
The B requirement of canopy between two sampling periods (t1 and t2) was: B content of new leaves produced between t1 and t2 plus B content accumulated by the older leaves between t1 and t2. It was also assumed that the B content in the canopy at the first sampling represented a continuous accumulation of B from the time of field planting since pruning of the leaves had not commenced then.
The B requirement of roots between two sampling periods (t1 and t2) was computed as follows:
B content at t2 – B content at t1 + a B content at t2
where a is the percentage of self-pruned roots. The a value for each time period was obtained from Jourdan and Rey (1997). It was also assumed that the root biomass continued to grow in the first 36 months after field planting with minimal self pruning following the logistic root model of Jourdan and Rey (1997).
The annual B requirements for fresh fruit bunches and male inflorescences were estimated based on their annual dry matter production multiplied by their average B concentration for the year.
Statistical analysis
Descriptive statistics, means and standard deviations, were calculated using Statistica Ver. 7.0 (StatSoft, 2004). Separate one factor analysis of variance (Anova) was computed for the B distribution within the vegetative components of the palm and the fresh fruit bunches. Non-linear regression was used to model the cummulative vegetative data using CurveExpert 1.38 (Hyams, 2001).
Oil Palm: Nutrition
Boron requirement and distribution in the oil palm (Elaeis guineensis Jacq.) and some implications on manuring practices
[addw2p name=”oilPalmNutrition”]
Oil palm (Elaeis guineensis Jacq.) is grown on more than 12 million hectares in the humid tropics mainly between latitude 10 ° N and 10 ° S. It contributes to about 27 % of the world’s vegetable oils and fats. Oil palm is the world’s most productive oil crop and requires a relatively high amount of B to sustain its growth and production despite being a monocotyledon (Shorrocks, 1997). In fact, it is one of the 16 plants regarded as most sensitive to B deficiency and highly responsive to B application (Shorrocks, 1997).
In Southeast Asia, the oil palm is mainly cultivated on the highly weathered Ultisols and Oxisols derived from granite, sandstones and shales. These soils have low soil B contents (Shorrocks, 1997) and therefore, B deficiency symptoms on the oil palm in various types of malformed, younger leaves are common particularly during drought. Boron deficiency causes premature lignification of the cell walls in the oil palm (Rajaratnam and Lowry, 1974) and under severe conditions, for example, at the little leaf stage, yield may decline by about 83% (Rajaratnam, 1973a). Thus, water-soluble B fertilizer such as Fertibor (15% B) is regularly applied at the rate of 1 to 3 kg B ha-1 yr-1 in the first six years after planting to prevent B deficiency in the oil palm.
Ng et al . (1968), working with the obsolete Dura planting materials, reported that B concentration in the oil palm canopy was similar to the stem. They did not find any trend in B concentrations between leaves of different ages and concluded that B may be mobile in the oil palm. In contrast, Rajaratnam (1972) using the current Tenera planting materials showed increasing B concentrations from the youngest to the oldest leaf suggesting that B is immobile in oil palm. However, they both agreed that B moves rapidly along the transpiration stream resulting in the accumulation of B at the tips of the oil palm leaf and leaflets.
K has been demonstrated to inhibit B uptake by oil palm (Rajaratnam, 1973b) whereas N fertilization may increase palm growth leading to low or deficient B concentration through dilution effect (Shorrocks, 1997). Since then, N and K fertilizer rates for the oil palm have increased substantially (Goh and Kee, 2000). It is therefore important to determine the B requirement of oil palm under current high fertilizer regimes. Tinker and Smilde (1963) postulated that the nutrient requirement of oil palm might be established by analysing the elements in the palm tissues because the bulk of the crop, and consequently its nutrient content, is large, and the long-term nutrient-supplying power of the soil is poor. The fraction of the total available nutrients held in the crop itself can then be considerable, and its nutrient requirements will be related to the amounts immobilized by the plants (Tinker and Smilde, 1963). These conditions are approached by the oil palm growing on soils with poor B supplying capacity as found in most of Southeast Asia. Despite this simple concept, the B requirements and distribution in the oil palm plantation has received little attention in the last three decades and has not been investigated for the current Tenera planting materials except in their canopy. Hence, this study was conducted with the following objectives:
-
To determine the B requirement of oil palm on an Oxisol at different palm ages
-
To investigate B mobility in oil palm using indirect method
-
To examine the distribution of B in an oil palm field at steady state on an Oxisol
Reference
Goh, K.J., Gan, H.H., Kee, K.K., Chew, P.S., and Teoh, K.C., (2007) Boron requirement and distribution in the oil palm (Elaeis guineensis Jacq.) and some implications on manuring practices. In: Xu. F., Goldbach H.E., Brown P.H., Bell R.W., Fujiwara T., Hunt C.D., Goldberg S. (Eds) Advances in Plant and Animal Boron Nutrition, Springer, The Netherlands: 189 – 202.
Note: The full list of references quoted in this article is available from the above paper.


